
The ReefRUV Global Network uses a relatively simple protocol designed to standardize the
sampling procedures of remote underwater videos (RUVs or videoplots) during field data
collection and lab analyses. This sampling technique can be applied in various habitats,
including coral reefs, rocky reefs, tide pools, freshwater clearwaters, and sandy substrates, as
well as in very shallow environments (up to 1 meter in depth). From the replicated videos,
standardized by time and area, it is possible to extract data on species richness, abundance,
biomass, feeding rates, animal consumption, and other behaviors of fish (e.g., cleaning,
agonistic interactions, etc.) and other marine organisms.
Our network is equipped to tackle macrobehavioral variations across space, time, and taxa. We
can study gradients of depth and reef complexity, ranging from local microhabitat scales to
latitudinal and temporal global scales. Additionally, we investigate co-occurrence patterns
using phylogenetic and functional approaches. Many sites were sampled 10–12 years ago, and
new sampling efforts may reveal differences that can be modeled for future scenarios.
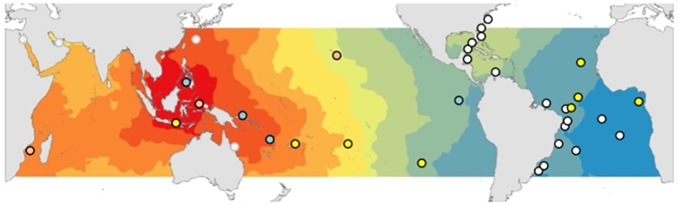
Sampling around the world. Metadata will soon be available.
Field Preparation
Before starting field activities, the person in charge must ensure that all necessary sampling
equipment is available. Essential items include:
 A charged digital camera for video recording. Camera resolution: 1080p at 60 fps, with A charged digital camera for video recording. Camera resolution: 1080p at 60 fps, with
Linear lens position or Medium field of view (FOV).
 A memory card with sufficient storage capacity; A memory card with sufficient storage capacity;
 Lead weights (2 kg) to secure the camera to the substrate; Lead weights (2 kg) to secure the camera to the substrate;
 A 1-meter graduated rope for marking the sampled area, ideally with markings every A 1-meter graduated rope for marking the sampled area, ideally with markings every
10 cm along its length to facilitate video analysis;
 A dive computer to record sampling time, depth, and temperature. A dive computer to record sampling time, depth, and temperature.
 10 cm bar (scale) to use when doing the photoquadrats. 10 cm bar (scale) to use when doing the photoquadrats.
Sampling
During fieldwork, the diver must ensure that the chosen sampling location is free of any
structures that could obstruct filming and compromise subsequent video analysis (see Figure).
Once a suitable sampling location is identified, the following steps should be followed:
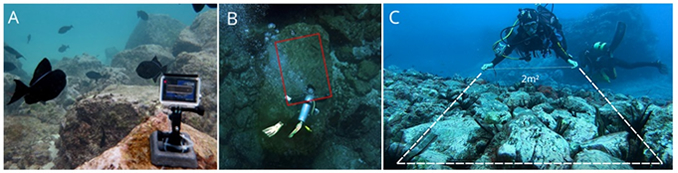
Demonstration of the sampling area demarcation process, showing: a) an example of the
sampled location, ensuring no obstructions in the filmed area; b) delineation of the sampling
area; c) demonstration of the lateral boundary demarcation.
a. Before installing the filming equipment, the diver should photograph the dive computer to
record the temperature and depth of the location.
b. After recording the temperature and depth data, the camera should be mounted on the
substrate using the weight. It should be positioned 1 to 1.5 meters from the sampling area,
ensuring the entire area is visible. Once positioned, ensure the camera is recording.
c. To demarcate the sampling area, the diver should use the graduated rope to measure 2
meters in length, 1 meter in height, and 1 meter in width. Begin by measuring the length,
followed by the height (at a distance of 2 meters from the camera), and finally, the width, to
delineate the sampling area (Figure).
d. To measure the width of the demarcated area, the diver should show the 1-meter mark to
the camera, holding it for a few seconds. Then, approach the camera in a straight line while
keeping the graduated rope taut (Figure C). This step is crucial as it defines the lateral
boundaries of the area analyzed in the video and facilitates future measurements of the sizes
of observed individuals.
e. After demarcating the area, the camera should record the sampling area for at least 15
continuous minutes. For analysis, only the central 10 minutes of the video will be considered,
discarding the first and last 2 minutes and 30 seconds to minimize the diver´s impact on the
sampled community.
f. Once the 15 minutes of sampling are complete, the diver will take 5 photoquadrats of the
benthos, using a 10 cm scale. After this, the camera can be removed and the diver can proceed
to the next location, following the same steps.
g. To avoid overlap, the sampling areas must be at least 3 meters apart.
Note: The height measured in each sampling area does not limit video analysis but may be
useful for studying species interactions at different heights from the reef bottom.
Selected Publications
Ferrari, D.S., Nunes, L.T., Mendes, T.C., Ferreira, C.E.L. & Floeter, S.R. (2024). Hyperdominance and
habitat composition drive reef fish foraging at Atlantic oceanic islands. Marine Ecology Progress
Series. doi: 10.3354/meps14483
Fontoura, L., Cantor, M., Longo, G.O., Bender, M.G., Bonaldo, R.M. & Floeter, S.R. (2020). The
macroecology of reef fish agonistic behaviour. Ecography, 43: 1278–1290, doi: 10.1111/ecog.05079
Inagaki, K.Y., Pennino, M.G., Floeter, S.R., Hay, M.E. & Longo, G.O. (2020). Trophic interactions will
expand geographically but be less intense as oceans warm. Global Change Biology. doi:
10.1111/gcb.15346
Nunes, L.T., Morais, R.A., Longo, G.O., Sabino, J. & Floeter, S.R. (2020). Habitat and community structure
modulate fish interactions in a neotropical clearwater river. Neotropical Ichthyology, 18(1):e190127.
doi: 10.1590/1982-0224-2019-0127
Longo, G.O., Hay, M.E., Ferreira, C.E.L. & Floeter, S.R. (2019). Trophic interactions across 61 degrees of
latitude in the Western Atlantic. Global Ecology and Biogeography, 28: 107–117. doi:
10.1111/geb.12806
Cantor, M., Longo, G.O., Fontoura, L., Quimbayo, J.P., Floeter, S.R. & Bender, M.G. (2018). Interaction
networks in tropical reefs.p. 141 –154. In: Dattilo, W. & Rico-Gray, V. (eds.) Ecological Networks.
Longo, G.O., Morais, R.A., ... & Floeter, S.R. (2015). Between-habitat variation of benthic cover, reef fish
assemblage and feeding pressure at the only atoll in South Atlantic: Rocas Atoll, NE Brazil. PLoS ONE
10(6): e0127176. doi: 10.1371/journal.pone.0127176
Longo, G.O., Ferreira, C.E.L. & Floeter, S. R. (2014). Herbivory drives large-scale spatial variation in reef
fish trophic interactions. Ecology and Evolution, 4(23): 4553–4566.
Partners and funding:

|




